Analytical equipment
The Chair of Chemical Engineering is currently equipped with the following analytical devices:
- Gas chromatography (GC) and mass spektrometry (MS)Hide
-

Gas chromatography (GC)
The gas chromatographic separation of components in a complex sample depends on different interactions between several substances and a specific column filling (stationary phase). The different chemical constituents pass in an inert gas stream (mobile phase) at different rates through the column (up to 100 m), depending on their various chemical and physical properties and their interaction with the column filling. As the chemicals exit the end of the column, they are detected and identified electronically. The function of the stationary phase in the column is to separate different components, causing each one to exit the column at a different time (retention time). Other parameters that can be used to alter the order or time of retention are the carrier gas flow rate and the temperature (up to 350 °C).
GC- equipment:
- GC 01: Varian CP 3800 with Saturn 2200 MS
- GC 02: Varian CP 3800 with FID-detector and PFPD-detector (sulfurselective detector)
- GC 03: Varian CP 3800 with FID-detector
- GC 04: HP5890 Series I with FID-detector, as online-GC
- GC 05: HP5890 Series I with FID-detector, as online-GC
- GC 06: Varian Micro-GC CP 4900 with WLD-detector, MS5- and PPQ-column
- GC 07: Varian Micro-GC CP 4900 with TCD-detector, PPQ-column
- GC 08: Varian CP 3800, LHA-analyzer with FID-detector
- GC 09: Perkin Elmer Clarus 500, Pona-analyser with FID-detector
- GC 10: Varian 450-GC with FID-detector
- GC 11: Bruker 450-GC with FID- and TCD-detector
- GC 12: Perkin Elmer Clarus 580 with FID-detector, as online-GC
- GC 13: Agilent 7890B (GC) with 5977A Series GC/MS (Single Quadrupole)
- GC 14: SRI 8610 with FID-detector
- GC 15: Agilent 7890B with FID- and TCD-detector, as online-GC
- GC 16: Agilent 7890B with FID-detector, as online-GC
Contact: Birgit Brunner
- ICP-OESHide
-
PerkinElmer Optima 7300 DV
ICP-OES
Inductively coupled plasma optical emission spectroscopy (ICP-OES) is an analytical technique used for the multi-element analysis of approximately 70 elements. A peristaltic pump delivers a dissolved sample into an analytical nebulizer where it is transformed into an aerosol and introduced directly into the very hot (about 10.000 K) radio frequency argon plasma.
The plasma is used to produce excited atoms and ions that emit electromagnetic radiation at wavelengths characteristical for a particular element. The intensity of this emission is proportional to the element concentration. Commercially available standards can be used to calibrate the ICP-OES, which makes it possible to perform highly quantitative analyses.Instrumental equipment: ICP-OES Optima 7300 DV (PerkinElmer)
application:
quantitative simultaneous multi-element determination of primary-, secondary- and trace components
elements: nearly all elements except H, N, O, noble gases, halogens sample requirement:
analyt must be in liquid form, solid samples have to be brought into solution with digestion methods, e.g. dissolution in hot acids field of application:
composition of catalysts, metal alloys sample quantity:
10-500 mg Contact: Birgit Brunner
- Elementary analysis (CHNS)Hide
-

EA 3000 (Fa. HEKAtech)
Elementary analysis of carbon, hydrogen, nitrogen, sulphur and oxygen
The elementaranalyser EA 3000 (company: HEKAtech) allows you to determine simultaneously the elements carbon, hydrogen, nitrogen and sulphur. With a second reactor the determination of oxygen is also possible.
Functional principle:
2-3 mg sample will be weighed into tin capsules and will be solubilized oxidative in an oxygen stream at approximately 990°C. The complete oxidation will be underwritten by a folowing wolframetrioxid catalyst. CO2, H2O and NOx are expected as products. The rest of the oxygen will be bound in a column with copper granulate and nitrogen oxides will be reduced to nitrogen. The gas mixture contains the analytgases CO2, H2O, N2 and SO2. High-purity helium is used as carrier material. The separation and quantification takes place in a gaschromatographe and the detection is done by a thermal conductivity detector.
Limit of determination: 0,05w-% (500ppm)
Contact: Birgit Brunner
- S-N-analyzer (liquids)Hide
-

Antek 9000NS
Schwefel/Stickstoff-Analyse
The Antek 9000NS analyser offers the parallel measurement of the total sulphur and nitrogen in liquid samples; the analyser is equipped with an auto sampler. The accuracy of analysis down ppb level for both elements. The sample volume for a single measurement ranges from 5 -15 µl, depending on the sulphur or nitrogen concentration of the substance. An accuracy of about ±2% is achieved.
The measuring principle is based on complete combustion of the sample at 1000 to 1100°C in an argon/oxygen-stream transforming sulphur to sulphur dioxide and nitrogen into nitrogen oxide. A membrane dryer removes the water from the resulting gas mixture to prevent condensation on the detectors avoiding biased results.
The sulphur dioxide concentration is determined by based on UV fluorescence; SO2 is excited by light and emits photons with a certain wave length. Nitrogen detection is based on chemiluminescence. For this purpose, nitrogen oxide formed during combustion is transformed by ozone, guided into the nitrogen-analyser, into excited nitrogen dioxide. On returning to the ground state, the electrons emit photons having small band width in their energy. The radiation, generated in the nitrogen- and sulphur-analyser, is amplified by a photomultiplier. The evaluation of the signals occurs computer-controlled by an adjustment with the density and by comparison with standard solutions.Contact: Birgit Brunner
- X-Ray Diffractometer (XRD)Hide
-

Bruker D8 Advance X-Ray Diffractometer
X-Ray Diffractometer (XRD)
With X-ray diffraction (XRD) structural and compositional information on materials can be gained. Thus, with an appropriate database for comparison, the phases in the material can be identified and quantified. Crystallite sizes can be determined also.
With the Bruker D8 Advance and Anton Paar XRK 900 measuring cell thus the structure and phase composition of a catalyst and in situ phase changes under reaction conditions (pressure to 10 bar, temperature to 900°C) can be examined.
Spezifications:
- X-Ray source: Cu-anode (2.2 kW)
- Geometries: Bragg-Brentano und parallel beam (Goebel mirror)
- Accessories: Anton Paar XRK 900 measuring cell for non-ambient (vacuum to 10 bar pressure, room temperature to 900 °C; reactive gases)
- Movable (X-Y-Z) sample stage for space-resolved measurement
- 1D-Lynxeye-Detektor
Contact: Dr.-Ing. Johannes Thiessen
- FT-IR (ATR)Hide
-

Nexus 470 from Thermo Nicolet
FT-IR (ATR)
The Fourier Transform Infrared Spectroscopy (FTIR) is a special alternative of the Infrared Spectroscopy (IR). The standard infrared spectrum is calculated from the Fourier-transformed interferogram, giving a spectrum in percent transmittance (%T) vs. light frequency (cm-1).
Operating mode:
in the system the mirrors are so arranged, that they build a so called Michelson-interferometer. The light coming from the IR-source is splitted by a beamsplitter in two single radiators. 50 % of the light is reflected to the fixed mirror and 50 % is transmitted to the moveable mirror. Light travels to each of the mirror and recombines at the beam splitter before passing through the sample and to the detector.
As the light intensity of the recombined beam is recorded at the detector, the moveable mirror travels towards the beamsplitter, producing an interferogram. As the moveable mirror travels, different frequencies are reflected in different ways. The summation of construcive and destructive interference over time makes an interferogram, from which a Fourier transform is used to calculate a spectrum.Accessories:
- 2 m gascell with ZnSe window with heater
- ATR-unit
- temperature controller
Contact: Birgit Brunner
- High Performance Liquid Chromatography (HPLC)Hide
-

Varian Pro Star
High Performance Liquid Chromatography (HPLC)
High-performance liquid chromatography (HPLC) is a form of liquid chromatography to separate compounds that are dissolved in solution. HPLC instruments consist of a reservoir of mobile phase, a pump, an injector, a separation column, and a detector. Compounds are separated by injecting a plug of the sample mixture onto the column. The different components in the mixture pass through the column at different rates due to differences in their partitioning behaviour between the mobile liquid phase and the stationary phase.
Application:
- Low volatile substance
- High polar or ionic substance (base, acid)
- Substance with high molecular mass (oligomers, polymers)
- Thermic instable and easy to pyrolyse substance
Instrument:
- Varian Pro Star
- UV/Vis Detektor 320
- Brechungsindex Detektor
Säule:
- Omnisphere C18 (reversed Phase)
- Length 25 cm
- ID 4,6 mm
- Size 5µm
- Pore 110 A
Contact: Birgit Brunner
- BET-surface-measurementHide
-

BET-surface-measurement
In fine-grained and/or porous solids the specific surface area is an important parameter in the characterisation of a material. Thus, process and quality control are largely predicted on measurements of the specific surface of adsorbents, catalysis, fillers, fertilisers, cement, metal powders, pigments, pharmaceuticals, ceramic materials and many other solids. Among the many and varied methods used to determine the specific surfaces areas of dispersed or porous solids, low-temperature gas adsorption according to Brunauer Emmet and Teller (BET) is of particular importance.
The background of the measurement is the determination of the adsorbed amount of a certain gas on the surface of a solid - within a constant temperature – as a function of pressure. Most generally nitrogen is used as an adsorption gas which attaches to the surfaces of solids by weak-interaction physisorption (van-der-waals-forces).Contact: Dr.-Ing. Johannes Thiessen
- ChemisorptionHide
-

Autochem II 2920 VG from Micromeritics
Dynamic Chemisorption Measurement and Temperature Programmed Reduction/Oxidation(TPR/TPO)
The catalytically active surface area of a catalyst often differs from the total surface area. The amount of active sites or the active surface area in a heterogeneous catalyst is a key characteristic, as other values, e.g. the dispersion or the diameter of the active (metallic) particles, can be calculated from that. Chemisorption measurements with gas phase probe molecules represent a relatively simple way to determine the catalytically active surface area. Beside the active surface area the strength of interaction between adsorbed molecules and adsorbent, and the temperature dependent degrees of reduction or oxidation of an active phase are important for the understanding of heterogeneous catalysis.
In this context, Temperature programmed desorption measurements make it possible to quantify the energy of desorption as measure for catalyst/reactant interactions. By TPR and TPO measurements reduction/oxidation temperatures and the degrees of reduction/oxidation can be determined.
The AutoChem II 2920 (Micromeritics) is a fully automated chemisorption analyser and can measure the temperature programmed reactions mentioned above, as well as metal surface areas by dynamic chemisorption measurements (pulse titration). Liquids at room temperature can be used as probe molecules due to a built – in vaporiser. Additionally, single- and multipoint BET surface areas can be determined by dynamic measurements.
Contact: Dr.-Ing. Johannes Thiessen
- Continious gasanalyzersHide
-

Gasanalyzer XSTREAM, Emerson
Continious gasanalyzers
15 general purpose continuous gasanalyzers measures up to five components simultaneously in various combinations.
These five components can be measured with NDIR/UV/VIS photometer, paramagnetic or electrochemical O2 and thermal conductivity.
Our gasanalyzers are equipped with CO, CO2, O2, CH4, H2, NO, NO2 or SO2 detectors.
Modern communication capabilities including web-browser functionality, RS 232 and analog output.Contact: Jörg Gerchau
- TG/DTAHide
-

TG/DTA 6300 , Seiko Instruments
Thermogravimetry and Differential Thermal Analysis (TG/DTA)
As is known, some physical or chemical processes can be characterized by a change in mass as well as evolution or absorption of heat. Both these characteristics can be defined by TG and DTA methods, in the course of which a sample is subjected to reaction conditions under either an isothermal regime or a programmed temperature run. Proceeding from the information obtained during such measurements, some kinetic parameters can be determined or estimated.
For carrying out such investigations, CVT uses TG/DTA 6300 device (Seiko Instruments Inc.) allowing us to expose samples to temperatures up to 1300°C with the temperature rise rate up to 20°C/min..
Among our investigations, there are nearly all typical physical and chemical processes such as:- evaporation
- drying
- melting
- oxidation
- reduction
- gasification
- other reactions between gas and solid.
As a representative example, the original data generated by TG/DTA 6300 in the reaction of a Ni-catalyst activation are shown in Figure.
Contact: Dr.-Ing. Johannes Thiessen
- Differential-Scanning-Calorimeter (DSC)Hide
-

DSC 204 Phoenix from Netsch with nitrogen cooling CC200L
DSC (Differential Scanning Calorimeter)
With the aid of the Differential Scanning Calorimetry you are able to determine the glass-, melting and decomposition temperatures as well as melting-, reactionenthalpy and heat capacity. The raw data can be used to identify the material and quantify it and allows a conclusion about the grade of the cristallinity and cross-linking. Measurement range: -180°C bis 700°C.
Instrument: DSC 204 Phoenix from Netsch with nitrogen cooling CC200L
Contact: Birgit Brunner
- Refracting indexHide
-

AR 2 , A.KRÜSS Optronic GmbH
Refracting index
Instrument: Abbe-Refractometer AR 2 from A.KRUESS Optronic GmbH
- Refractive Index Range: 1,300 - 1,700 nD
- Resolution: 0,001 nD
- Brix: 0,5 %
- Electronic heating and cooling
The instrument measures the refracting index nD, the concentration in % and the dispersion nF-nC of transparent and opaque liquids and solids. In combination with a thermostat the instrument measures the refraction index in a temperature range of 0° to 70°C.
The optical composition of this refractometer makes it possible to identify precise the critical angel with a thin layer of liquid, which is located between two glass prisms.
Refractive index and mean dispersions belong to one of the important optical constants in substance, which can be used to check in substance optical performance, purity, concentration and dispersion etc.
The Abbe-Refractometer is suitable for the following determinations:
- Determination of concentration
- Determination of mixing ratio
- Purity control
- Manufactoring control of products
Contact: Birgit Brunner
- Karl-Fischer-CoulometerHide
-

KF-Coulometer 831, Metrohm
Water content determination by Karl-Fischer
The Karl-Fischer titration is a method to determine the water content of technical products, foods, chemical substances, etc. (Karl Fischer, German chemist, 1901 – 1958)
We use a KF-Coulometer 831 (Metrohm) for the coulometrical water determination by Karl Fischer. The measuring cell consists of an anode and a cathode room which are separated by a diaphragm. The liquid sample is injected in the anode room through a septum.
In the KF titration one uses the redox reaction between iodine and sulfur dioxide, which proceeds only in presence of water:
I2+SO2+2H2O -> 2I-+2H++SO3
Solvent is anhydrous methanol which reacts with SO3 to sulfuric acid ether. The protons are neutralized with a Bronsted base. The reaction is finished, if an iodine excess is reached. The water content of the sample can be read directly form the display in µg water resp. in ppm or percent.
Range of application: 10 µg to 200mg
Contact: Birgit Brunner
- Melting point instrumentHide
-

Büchi Melting Point B-540
Melting point instrument
Instrument: Büchi Melting Point B-540
The Melting-Point-Instrument B-540 is an instrument to determine the melting- and boiling point from room temperature up to 400°C
Three different samples can be analysed simultaneously. The detection of the values is done by visual study of the sample by the operator.
Contact: Birgit Brunner
- Magnetic suspension balances (MSB)Hide
-
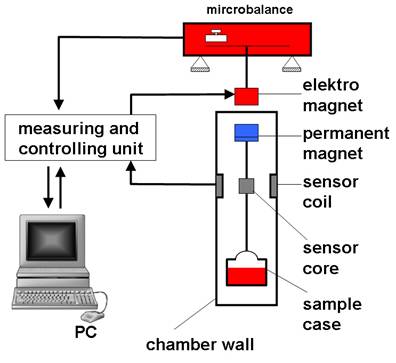
Magnetic suspension balances (MSB)
Magnetic suspension balances (MSB) allow obtaining extremely precise measurements of mass changes which act on samples under defined environments (pressure, temperature, aggressive atmospheres etc.). These measurements enable to determine transport quantities and state quantities quickly and accurately, (sorption, diffusion, surface, tension, density).
In this method, the measuring force is transmitted contactless via magnetic coupling from the measuring chamber to a microbalance, which is located outside the chamber under ambient atmospheric conditions. The suspension magnet, which is used for transmitting the force, consists of a permanent magnet inside of the measuring chamber; a sensor core and an electromagnet outside the chamber attached to the microbalance
The atmospheric disjunction of microbalance and sample in the MSB allows the measurement of weight changes of samples under severe conditions (T< 250 °C, p < 400 bar). Because of the very high precision (- 0.01 mg) of the microbalance, this technique is particularly suitable to study the sorption of gases in liquids or solids. Therefore an interesting field for application of a MSB is the determination of sorption and diffusion properties of gases and gas components in ionic liquids (ILs) or in polymers.
Selected publications:
- Dreisbach, F.; Losch, H. W.: Magnetic suspension balance for simultaneous measurement of a sample and the density of the measuring fluid. of Thermal Analysis and Calorimetry (2000), 62(2), 515-521.
- Dreisbach, Frieder; Seif A. H., Reza; Loesch, Hans Wilhelm: Measuring techniques for gas-phase adsorption equilibria. Chemie Ingenieur Technik (2002), 74(10), 1353-1366 (in german).
- Kern, C.; Jess, A.; Altstaedt, V.; Langenfelder, D.; Woellecke, F.: Measurement of solubility and mass transfer of CO2 in polymeric materials using a magnetic suspension weighing device. Chemie Ingenieur Technik (2004), 76(9), 1352 (in german).
- Kern, C.; Jess, A.; Korth, W.: Use of a magnetic suspension weighing device for determination of gas solubility and mass transfer in ionic liquids. Chemie Ingenieur Technik (2004), 76(9), 1351-1352 (in german).
- Kern, C.; Korth, W., Jess, A.: Determination of Gas Sorption and Mass Transfer in Ionic Liquids by Means of a Magnetic Suspension Balance. 1st International Congress on Ionic Liquids (COIL), 19.-22.6. 2005, Salzburg /Austria.
Contact: Dr.-Ing. C. Kern
- NMR spectroscopyHide
-
Spinsolve Carbon 60 MHz Benchtop NMR (1H, 19F, 13C)
NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy, is a spectroscopic technique to observe local magnetic fields around atomic nuclei. NMR spectroscopy provides detailed information about the structure, dynamics, reaction state, and chemical environment of molecules.
The sample is placed in a magnetic field and the NMR signal is produced by excitation of the nuclei sample with radio waves into nuclear magnetic resonance, which is detected with sensitive radio receivers. The intramolecular magnetic field around an atom in a molecule changes the resonance frequency, thus giving access to details of the electronic structure of a molecule and its individual functional groups. The most common types of NMR are proton (1H) and carbon-13 (13C) NMR spectroscopy, but it is applicable to any kind of sample that contains nuclei possessing spin.
Contact: Dr.-Ing. Wolfgang Korth
- Bomb calorimeterHide
-

Bomb calorimeter IKA C200
Bomb calorimeter
The gross calorific value is determined in a bomb calorimeter (IKA C200) in an oxygen atmosphere and at a pressure of 30 bar. A bomb calorimeter consists of an enclosure in which the reaction takes place. The substance to be determined is placed in the bomb and electrical energie is used to ignite the sample.The surrounded water absorbs the heat of the reaction and thus increases in temperature. Measurement of this temperature rise and a knowledge of the weight and heat characteristics of the container and liquid permits the total amount of heat generated to be calculated.
Contact: Birgit Brunner


